Our Research
Biopolymers
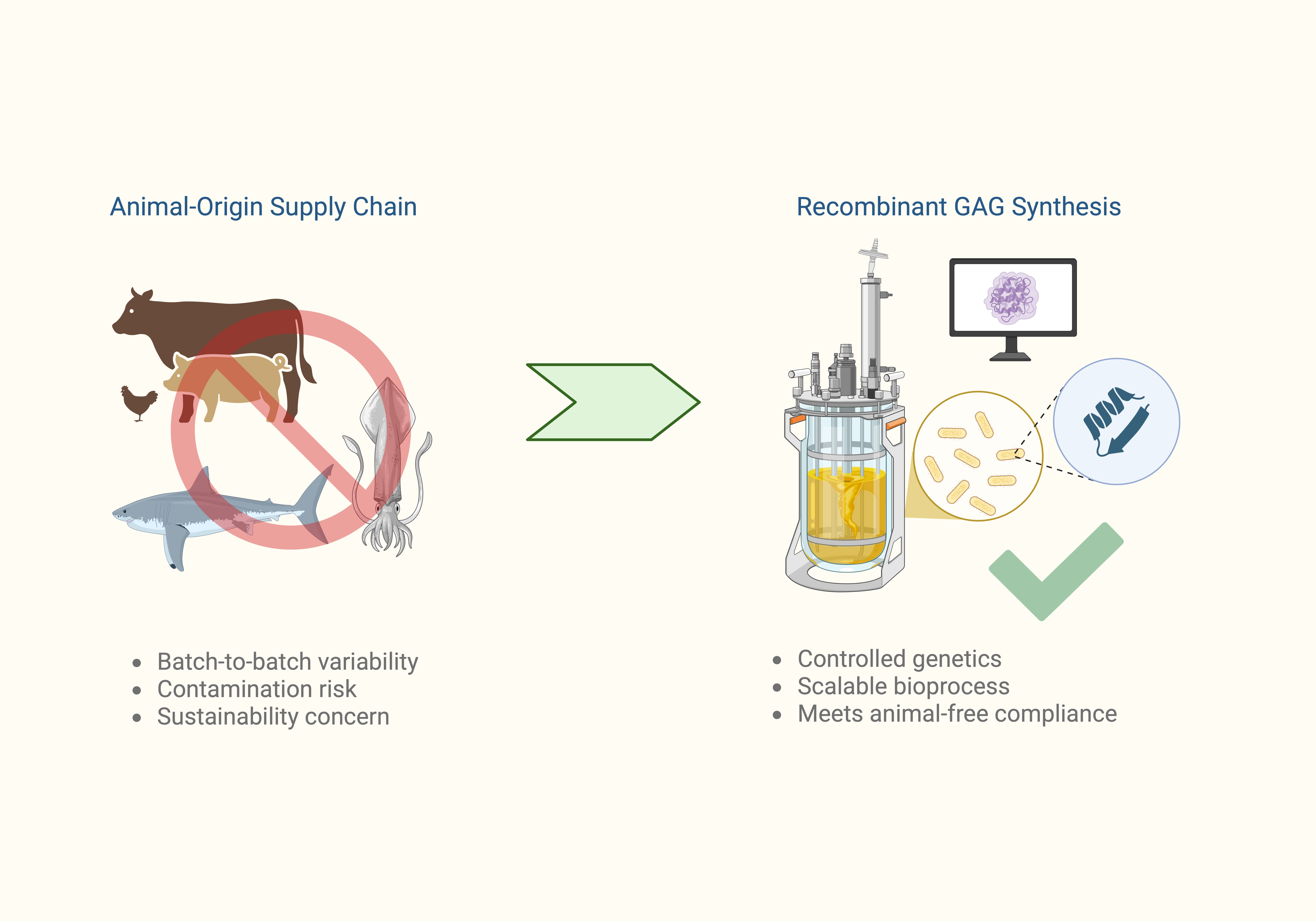
Recombinant Production of Chondroitin Sulfate
Chondroitin sulfate is an important biomolecule widely used in medical and therapeutic applications, particularly for joint health and tissue repair. Traditionally sourced from animal cartilage, its production faces challenges related to sustainability, safety, and consistency. Our lab is applying synthetic biology and protein engineering to develop microbial platforms for the sustainable and scalable production of CS.
- Engineer and characterize sulfotransferases to control CS sulfation patterns and enhance functional specificity
- Apply tailored CS molecules in biomedical applications, including potential therapies for neurodegenerative diseases such as Alzheimer’s
- Advance cell-based biomanufacturing platforms to produce glycoaminoglycans with improved safety and tunability

Recombinant Silk Production
Silk combines exceptional strength and flexibility with biocompatibility and biodegradability, making it ideal for textiles, tissue engineering, and sustainable materials. We focus on designing novel recombinant silk proteins to meet the demands of modern materials science and biotechnology.
- Eliminate reliance on animal-derived materials to create sustainable and ethically produced biomolecules
- Genetically engineer microbial hosts for improved expression, yield, and assembly of these recombinant silk proteins

Gamma-PGA
Poly(γ-glutamic acid) (γ-PGA) is a biodegradable, water-soluble biopolymer with applications across agriculture, medicine, food, and cosmetics, but current production methods lack sustainability. We engineer novel bacterial strains that convert unconventional carbon sources, including plastic-derived aromatics, into γ-PGA to create value-added pathways for waste valorization and sustainable biopolymer production.
- Engineer metabolic pathways in bacteria to efficiently convert plastic-derived aromatics and other waste streams into γ-PGA
- Integrate plastic upcycling with biopolymer production to create circular economy solutions for waste management

Chromoproteins
Chromoproteins offer biodegradable, renewable alternatives to synthetic dyes that harm the environment through toxic runoff and excessive water use. We engineer durable chromoproteins for sustainable coloration across textile, cosmetic, food, and paper industries.
- Engineer chromoproteins with enhanced colorfastness and stability for industrial applications
- Develop scalable microbial production systems for cost-effective chromoprotein manufacturing
Plastic Upcycling
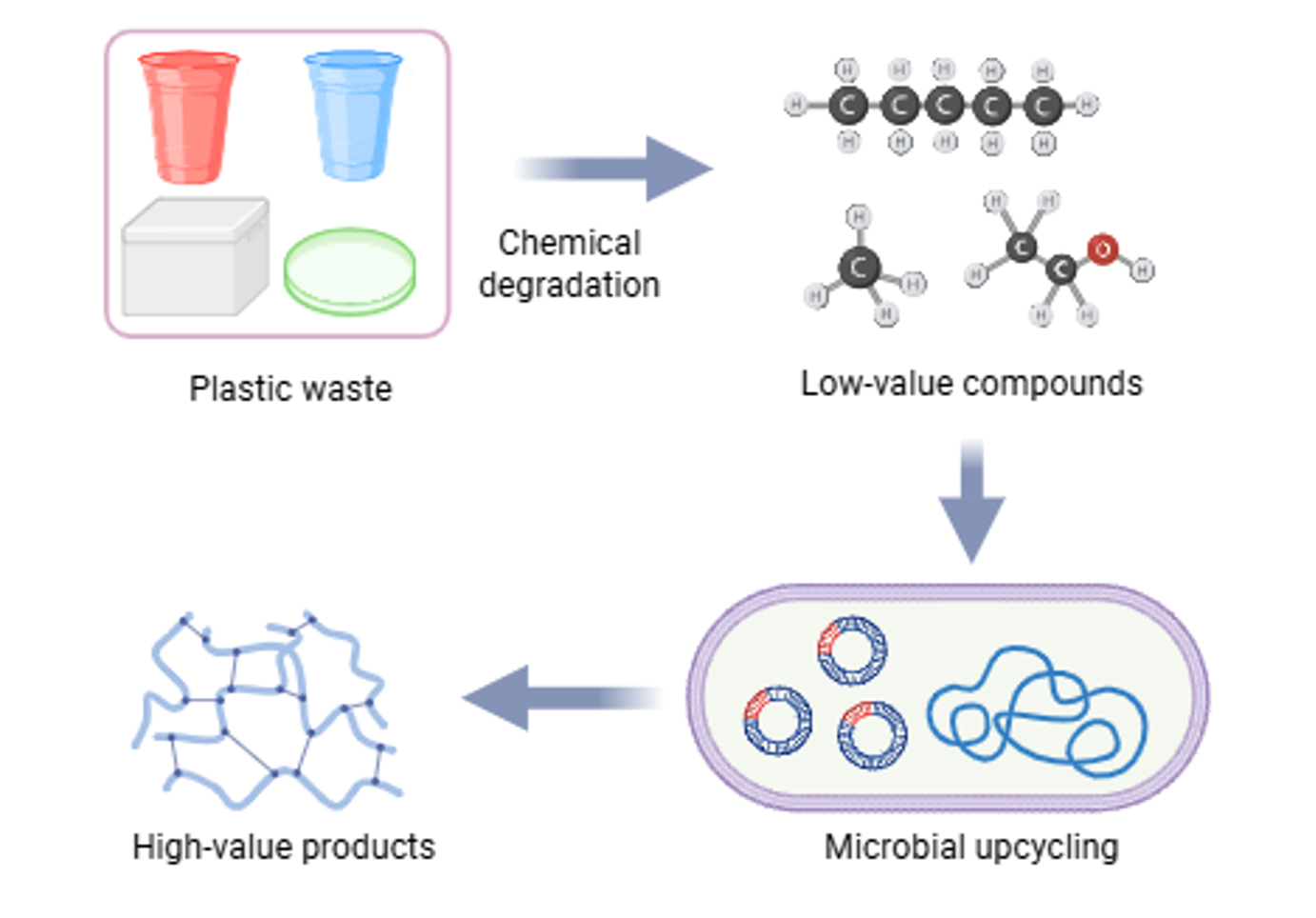
Plastic pollution presents a critical environmental challenge with millions of tons accumulating in ecosystems annually, while conventional recycling remains inefficient for many plastic types. We develop sustainable upcycling strategies that convert non-biodegradable plastics like polystyrene and PET into high-value bioproducts through integrated chemical and biological processes.
- Combine plastic depolymerization with microbial fermentation to transform plastic-derived compounds into functional materials
- Engineer pathways that convert plastic waste into biopolymers and platform chemicals to support circular economy solutions
Genome Editing
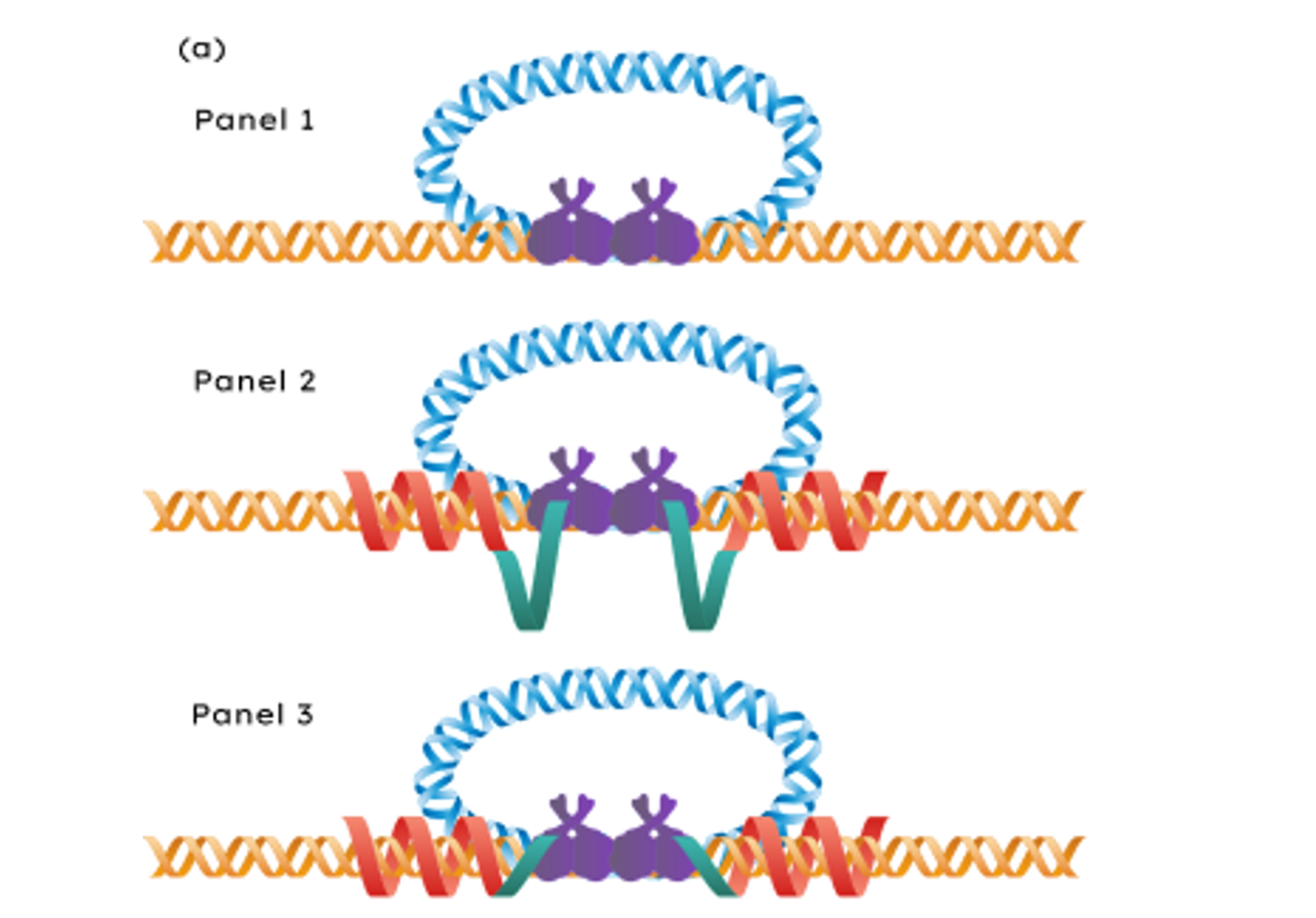
Current gene editing tools face critical limitations - CRISPR struggles with non-dividing cells and large DNA insertions, while lentiviral vectors lack insertion specificity despite excellent integration efficiency. We develop a novel platform that combines viral delivery precision with programmable targeting to enable efficient, site-specific gene editing across diverse cell types.
- Engineer fusion proteins combining HIV integrase with programmable targeting domains for site-specific integration
- Develop modified lentiviral architectures that ensure proper assembly and delivery of targeting components to both dividing and non-dividing cells
Bioengineering Scale-Up
Fermentation
Recombinant microbial fermentation is a powerful technology used to produce a wide range of products, including therapeutic proteins, industrial enzymes, and bio-based chemicals. Microorganisms such as bacteria and yeast are genetically engineered to express target genes, enabling high-yield biosynthesis in controlled bioreactor environments.
- Optimize fermentation parameters such as pH, temperature, oxygen levels, and nutrient feed strategies to support cell growth and maximize product output
- Apply expertise in microbial host engineering and bioprocess development to deliver robust, cost-effective, and scalable biotechnological solutions
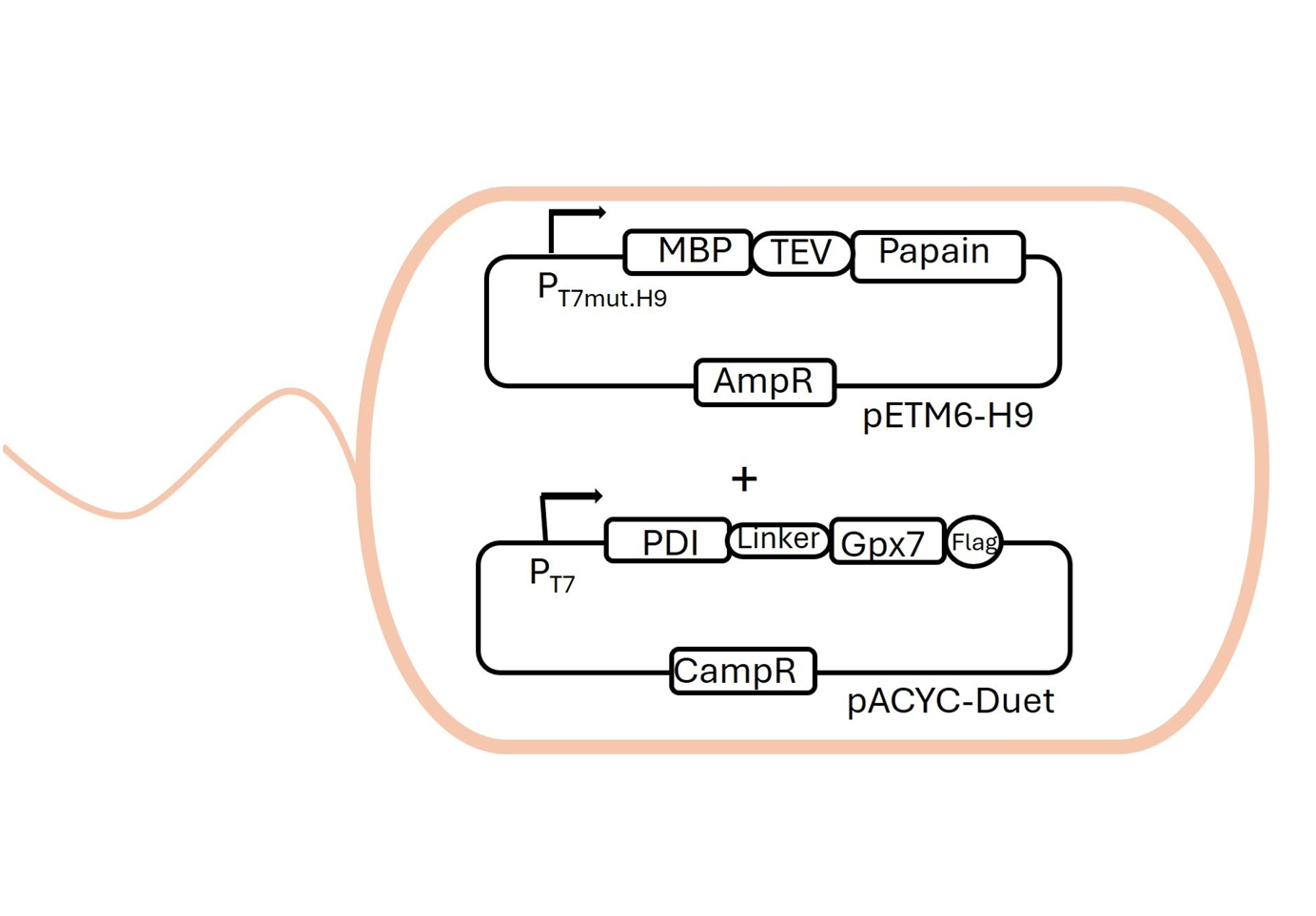
Papain
Papain, a cysteine-like protease, has extensive applications across industries such as food, chemical, pharmaceutical, and polymer manufacturing.
- Engineer proteases for efficient and valuable peptide synthesis
- Prodive insights into recombinant papain microbial production that can lead to an industrially viable production strain.
Cell-free Metabolic Engineering
Commercial chemical production increasingly relies on engineered biological systems for their stereoselectivity and sustainability advantages, but limited intracellular accumulation of target metabolites often restricts optimal titers in living cells. We develop cell-free metabolic engineering platforms that overcome these constraints to achieve significantly higher compound yields for biocatalytic synthesis.
- Optimize microbial hosts to effectively channel carbon flux towards desired metabolic pathways
- Characterize and manipulate metabolic fluxes within cell-free systems to maximize product formation and yields
Textile Engineering

Current fiber industries face a sustainability crisis due to reliance on fossil-based materials, while natural fibers struggle to compete with synthetic alternatives. We develop "bioalloying" approaches that engineer molecular-level synergies between biobased materials to create multi-component fibers with enhanced properties and consistent performance
- Engineer molecular interactions between natural fiber constituents to create novel property combinations
- Develop bioalloying strategies that provide tunable fiber properties despite variability in natural feedstocks